

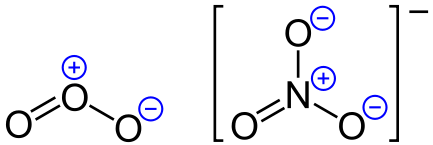
O2 and O4 are identical, so their formal charges are 0 respectively. The formal charge on O2 = 6 – 4 – 2 = 6 – 6 = 0 In the same way, the formal charges on O2 and O4 are the same due to the identical nature of the oxygen atoms. Now, we will find, the formal charge on the O1 atom.įormal charge on O1 = 6 – 6 – 1 = 6 – 7 = -1Īs we discussed, O1 and O3 are identical so, formal charges are -1 We need to find the formal charges of O1, O2, O3, and O4īut we noticed here that the formal charges on O1 and O3 are the same because the O1 and O3 are identical atoms.
#FORMAL CHARGE FORMULA HOW TO#
How to calculate the formal charge?Ī formal charge of an atom can be found with the help of the following formula. Because of the partial charge of the atom, however, there are unit exceptions. They will even be used for qualitative comparisons between different resonance structures of the identical molecule.
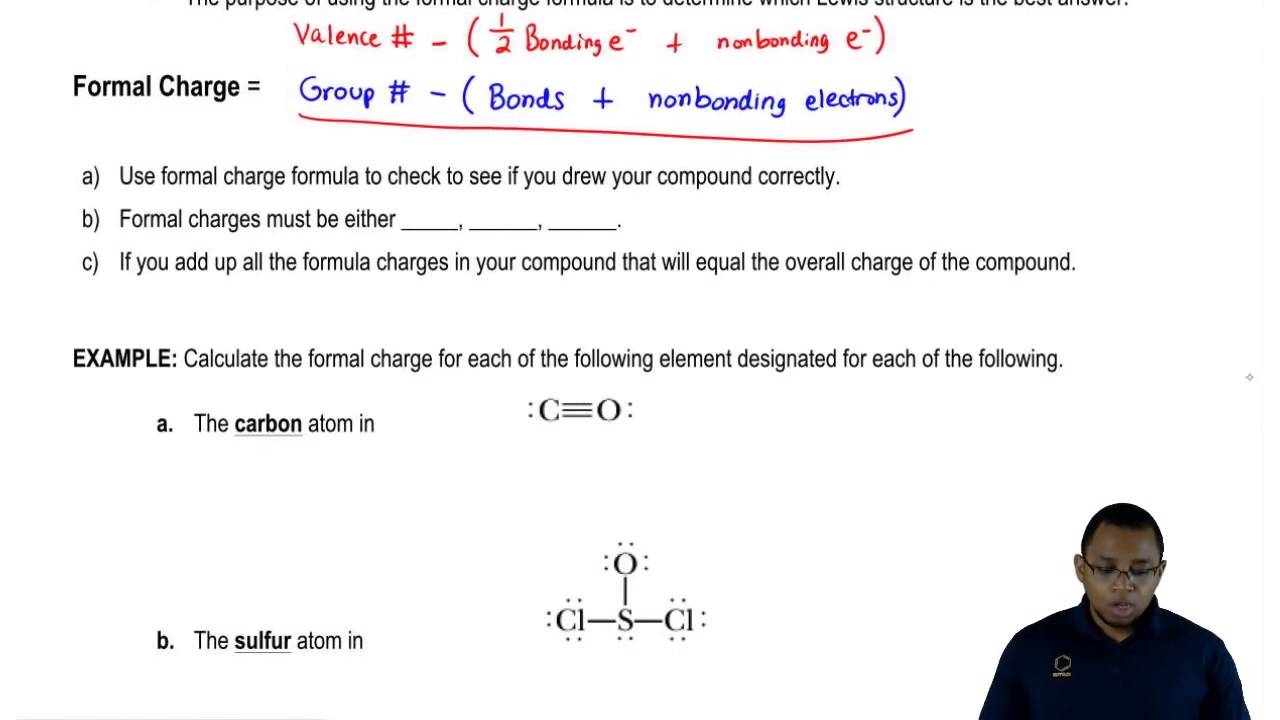
However, they don’t have any intrinsic physical which means. It’s simply less the number of formal charges more the stable structure. How we can determine the stability of structure based on the formal charge. So, what is the need for a formal charge? Formal charge decide which lewis acid is the best to structure.įormal charges area unit calculated by employing a set of rules and area unit helpful for accounting for the electrons once writing a reaction mechanism. The formal charge is the charge denoted to an atom in a molecule.


 0 kommentar(er)
0 kommentar(er)
